G.Patton
Expert
- Joined
- Jul 5, 2021
- Messages
- 3,098
- Solutions
- 3
- Reaction score
- 3,563
- Points
- 113
- Deals
- 1
Introduction

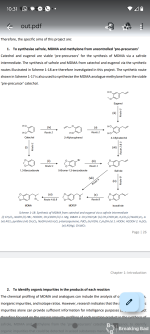
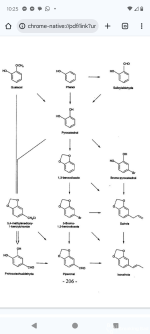
These precursors, are controlled or regulated substances in many jurisdictions. The use of uncontrolled precursors therefore offers clandestine laboratory operators a strategy to reduce the risk associated with detection. Catechol (1) is a common chemical reagent that is synthesized on an industrial scale with applications in the synthesis of fragrances, pesticides, drugs and dyes. Diversion of Catechol into illicit activities is therefore highly feasible. Safrole (4), a common starting material for MDMA production, is a natural product obtained from sassafras oil and is also used for the industrial production of fragrances, flavours and some insecticides. The synthesis of safrole from synthetic precursors, including catechol, has been investigated as a means to reduce the reliance upon variable natural sources. This paper presents a synthetic method of producing MDP2P (6) from catechol via Safrole, the reaction pathways shown in scheme below. The synthesis of Safrole is represented by a reaction with two different conditions and slightly different yields. The synthesis of MDP2P from safrole was performed via the two most common methods used in clandestine laboratories – Wacker oxidation of safrole (Route 1) with 78% yield (of this reaction) and the isomerisation of safrole and peracid oxidation and acid dehydration of isosafrole (Route 2) with 57% yield (of both route 2 reactions).
Equipment and glassware:
- 100 mL and 1 L three necked round bottom flasks;
- Reflux condenser;
- Heated magneticstirrer;
- Retort stand and clamp for securing apparatus;
- Laboratory grade thermometer (10 °C to 200 °C) with flask adapter;
- Rotary evaporator;
- Vacuum source;
- Water bath;
- Glass rod;
- 500 mL x2; 200 mL x2; 100 mL x2 Beakers;
- 250 mL x2 and 100 mL Erlenmeyer flasks;
- 1 L Buchner flask and funnel;
- 1 L Separating funnel, 250 mL for [1];
- Conventional funnel;
- 100 mL Drip funnel;
- 10 mL Plastic or glass syringe.
- Method [1]
- Nitrogen or Argon balloon 10-20 L (1 atm) is enough;
- 10 mL Pear-shaped flask.
- Method [2]
- Oil bath;
- 500 mL x2 Round bottom flasks;
- (Route 1)
- 10 mL Pear-shaped flask.
- (Route 2)
- 50 mL pear-shaped flask.
Reagents:
- Catechol 20.0 g, 182 mmol;
- Sodium hydroxide 100 g;
- Dimethyl sulfoxide (DMSO) 200 mL;
- Dichloromethane (CH2Cl2) 40 mL, 626 mL;
- Distilled water (H20) 1.5 L;
- Diethyl ether (Et2O) 1140 mL;
- Sodium sulphate (Na2SO4) or Magnesium sulphate (MgSO4) anhydrous ~200 g;
- Glacial acetic acid 2.6 mL, 45 mmol;
- Methanol (MeOH) 16 mL;
- Hydrobromic acid (HBr) 6.0 mL, 8.9 M, 53 mmol;
- Hydrogen peroxide (H2O2) 6.0 mL, 9.9 M, 59 mmol;
- Sodium bisulfite (NaHSO3) solution 10 mL 10% aq. [Sodium bicarbonate sol. can be used instead of this];
- Sodium bicarbonate (NaHCO3) 20 g;
- Hydrochloric acid (HCl) conc. ~60 mL
- Methanol 10 mL;
- Sodium chloride (NaCl) 30 g;
- Allyl bromide 40 mL (0.47 moles) [2] or 4.0 mL, 46 mmol [1];
- Tetrahydrofuran (THF) 170 mL anhydrous;
- Magnesium (Mg) 0.60 g, 25 mmol [1] or 10-11g [2].
- Method [1]
- DIBAH 1.5 M solution in cyclohexane (0.10 mL, 150 mmol);
- Ammonium chloride (NH4Cl) saturated solution, 20 mL.
- Method [2]
- Iodine crystal (I2);
- Dibromomethane (CH2Br2) 2 mL;
- Copper(I)iodide 0.5 g;
- Ammonia solution (NH4OH) 80 mL 25%;
- Diethyl ether 800 mL.
- (Route 1)
- Palladium (II) chloride (PdCl2) 12 mg, 68 mmol;
- p-Benzoquinone 0.85 g, 7.9 mmol.
- (Route 2)
- Potassium hydroxide (KOH) 3 M aq. solution;
- 1-Butanol 10 mL, 30 mmol;
- Hydrogen peroxide (H2O2) 2.0 mL, 9.9 M aq. solution, 20 mmol;
- Formic acid 10 mL, 23.6 M, 240 mol;
- Acetone 6 mL;
- Sulphuric acid (H2SO4) 10 mL 2.8 M aq.
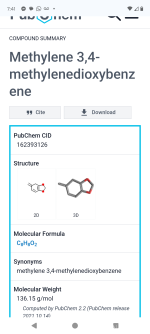
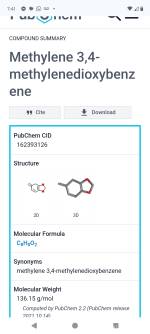
1-(2H-1,3-Benzodioxol-5-yl)propan-2-one:
Boiling Point: 283 - 285 °C at 760 mm Hg, 156 °C at 11 mm Hg;
Melting Point: 87 - 88 °C;
Molecular Weight: 178.185 g/mole;
Density: 1.211 g/mL;
CAS Number: 4676-39-5.
Procedure
1,3-benzodioxole (2)1 L three necked round bottom flasks with reflux condenser, magnetic/top stirrer and thermometer was filled with catechol (1) (20.0 g, 182 mmol) and an aqueous solution of sodium hydroxide (30 mL, 19.4 M, 582 mmol) which were dissolved in 200 mL of dimethyl sulfoxide. The resultant green solution was heated to 90–100°С. Dichloromethane (40 mL, 626 mL) was added drop wise to the solution, which was heated under reflux at 90–100°С for 4 h. The mixture was allowed to cool and 200 mL of water was added. The mixture was decanted, and the product was extracted with diethyl ether (3 x 200 mL). The diethyl ether extracts were washed with 3 x 200 mL of water, dried over anhydrous sodium sulphate and decanted. Solvent was removed with a rotary evaporator, producing a light-brown oil. Yield: 14.8 g (66.7%).
5-bromo-1,3-benzodioxole (3)
1,3-Benzodioxole (2) (6.00 mL, 52.2 mmol) was dissolved in a mixture of glacial acetic acid (2.6 mL, 45 mmol), 16 mL of methanol, and 2 mL of water in 100 mL three necked round bottom flasks with reflux condenser, magnetic stirrer and thermometer was. Hydrobromic acid (6.0 mL, 8.9 M, 53 mmol) was then added dropwise to the solution, ensuring that the temperature remained below 25°С. The solution was heated to approximately 35°С, and hydrogen peroxide (6.0 mL, 9.9 M, 59 mmol) was added drop wise, ensuring that the temperature did not exceed 50°С. The resulting solution was stirred at 40–50°С for 3 h and allowed to cool. The red organic layer was extracted with diethyl ether (1 x 40 mL) and washed with 10 mL of aqueous 10% sodium bisulfite solution. The ether extracts were dried over anhydrous sodium sulphate, decanted and the solvent removed with a rotary evaporator, producing an orange oil. Yield: 8.95 g (85.2%).Safrole [5-(2-Propen-1-yl)-1,3-benzodioxole] (4)
[Method 1]
The following Grignard reaction was conducted using dry pear-shaped flask 10 ml under nitrogen. Magnesium (0.60 g, 25 mmol), 5-bromo-1,3-benzodioxole (3) (0.40 mL, 3.3 mmol) and a 1.5 M solution of DIBAH in cyclohexane (0.10 mL, 150 mmol) were stirred in 20 mL of anhydrous THF. Additional 5-bromo-1,3-benzodioxole (3) (2.60 mL, 21.5 mmol) was added drop wise and the mixture was stirred for 2 h. The solution was removed via syringe and added dropwise to allyl bromide (4.0 mL, 46 mmol) contained in an ice bath. The solution was stirred for 24 h and the reaction quenched by the addition of 20 mL of water and 20 mL of saturated ammonium chloride. The product was extracted into 3 x 80 mL of diethyl ether and washed with 3 x 100 mL of water. The ether extracts were dried over anhydrous sodium sulphate, decanted, and the solvent removed with a rotary evaporator, producing a brown oil. Yield: 3.30 g (82.1%).
[Method 1]
The following Grignard reaction was conducted using dry pear-shaped flask 10 ml under nitrogen. Magnesium (0.60 g, 25 mmol), 5-bromo-1,3-benzodioxole (3) (0.40 mL, 3.3 mmol) and a 1.5 M solution of DIBAH in cyclohexane (0.10 mL, 150 mmol) were stirred in 20 mL of anhydrous THF. Additional 5-bromo-1,3-benzodioxole (3) (2.60 mL, 21.5 mmol) was added drop wise and the mixture was stirred for 2 h. The solution was removed via syringe and added dropwise to allyl bromide (4.0 mL, 46 mmol) contained in an ice bath. The solution was stirred for 24 h and the reaction quenched by the addition of 20 mL of water and 20 mL of saturated ammonium chloride. The product was extracted into 3 x 80 mL of diethyl ether and washed with 3 x 100 mL of water. The ether extracts were dried over anhydrous sodium sulphate, decanted, and the solvent removed with a rotary evaporator, producing a brown oil. Yield: 3.30 g (82.1%).
[Method 2]
In a 500 mL flask (immersed in a magnetic stirrer / oil bath) are placed 10-11 g magnesium turnings, and 150 mL tetrahydrofuran (freshly distilled from sodium). After the addition of a little iodine crystal and 2 mL dibromomethane to start the Grignard reaction, the 72 g of 5-bromo-1,3-benzodioxole (3) are added to maintain gently reflux. To start up, heating of the bath to 50 °C is recommended. After the addition, which takes about 60 min., the whole is stirred and refluxed 1 h, and the brown liquid is rapidly decanted to a very dry 500 mL flask with dropping funnel and reflux condenser. The magnesium turnings are washed with additional 20 mL dry THF, the washing is added to the Grignard solution. A little (0.5 g) copper(I)iodide is added, and with cooling in an ice-bath, 40 mL (0.47 moles) allyl bromide are added dropwise, the internal temperature should not exceed 40 °C. After standing overnight, followed by 1 hr of refluxing, the reaction mixture is suspended in a solution of 20 mL 37% hydrochloric acid in 500 mL water and this is added to 80 mL 25% ammonia, and the solution is steam distilled as above. After collecting 2 L distillate, the distillate is acidified to congo red (pH 4) with hydrochloric acid, saturated with table salt, and extracted with 4x200 mL ether. The combined extracts are dried with sodium hydroxide (or MgSO4), evaporated (rotavap), and the residue taken up in ether, and washed thoroughly with sodium hydroxide. After drying (sodium sulfate), the drying agent is filtered, washed with 20 mL ether, and the combined extracts are evaporated. The residue is vacuum distilled, 39 g (67% of theory) of safrole, boiling at 120-130 °C (20-25 mm Hg), are obtained. Colourless and typically smelling oil.
MDP2P (6) (Route 1)
Safrole (1.00 g, 6.17 mmol) was dissolved in 1 mL of methanol in 10 mL pear-shaped flask and added dropwise to a mixture of palladium (II) chloride (12 mg, 68 mmol), p-benzoquinone (0.85 g, 7.9 mmol), 5 mL of methanol and 0.5 mL of water. The resulting mixture was stirred for 3 h and filtered. To the filtrate, 10 mL of 3.2 M hydrochloric acid was added. The product was extracted with 3x 20 mL of dichloromethane and washed with 2 x 20 mL of a saturated sodium bicarbonate solution, 2x 20 mL of 1.3 M sodium hydroxide and 2 x 20 mL of brine. The organic extracts were dried over anhydrous sodium (or magnesium) sulphate, decanted and the solvent removed with a rotary evaporator, producing a brown oil. Yield: 857 mg (78.0 %).
In a 500 mL flask (immersed in a magnetic stirrer / oil bath) are placed 10-11 g magnesium turnings, and 150 mL tetrahydrofuran (freshly distilled from sodium). After the addition of a little iodine crystal and 2 mL dibromomethane to start the Grignard reaction, the 72 g of 5-bromo-1,3-benzodioxole (3) are added to maintain gently reflux. To start up, heating of the bath to 50 °C is recommended. After the addition, which takes about 60 min., the whole is stirred and refluxed 1 h, and the brown liquid is rapidly decanted to a very dry 500 mL flask with dropping funnel and reflux condenser. The magnesium turnings are washed with additional 20 mL dry THF, the washing is added to the Grignard solution. A little (0.5 g) copper(I)iodide is added, and with cooling in an ice-bath, 40 mL (0.47 moles) allyl bromide are added dropwise, the internal temperature should not exceed 40 °C. After standing overnight, followed by 1 hr of refluxing, the reaction mixture is suspended in a solution of 20 mL 37% hydrochloric acid in 500 mL water and this is added to 80 mL 25% ammonia, and the solution is steam distilled as above. After collecting 2 L distillate, the distillate is acidified to congo red (pH 4) with hydrochloric acid, saturated with table salt, and extracted with 4x200 mL ether. The combined extracts are dried with sodium hydroxide (or MgSO4), evaporated (rotavap), and the residue taken up in ether, and washed thoroughly with sodium hydroxide. After drying (sodium sulfate), the drying agent is filtered, washed with 20 mL ether, and the combined extracts are evaporated. The residue is vacuum distilled, 39 g (67% of theory) of safrole, boiling at 120-130 °C (20-25 mm Hg), are obtained. Colourless and typically smelling oil.
MDP2P (6) (Route 1)
Safrole (1.00 g, 6.17 mmol) was dissolved in 1 mL of methanol in 10 mL pear-shaped flask and added dropwise to a mixture of palladium (II) chloride (12 mg, 68 mmol), p-benzoquinone (0.85 g, 7.9 mmol), 5 mL of methanol and 0.5 mL of water. The resulting mixture was stirred for 3 h and filtered. To the filtrate, 10 mL of 3.2 M hydrochloric acid was added. The product was extracted with 3x 20 mL of dichloromethane and washed with 2 x 20 mL of a saturated sodium bicarbonate solution, 2x 20 mL of 1.3 M sodium hydroxide and 2 x 20 mL of brine. The organic extracts were dried over anhydrous sodium (or magnesium) sulphate, decanted and the solvent removed with a rotary evaporator, producing a brown oil. Yield: 857 mg (78.0 %).
Synthesis of isosafrole (5) (Route 2)
Safrole (1.40 g, 8.63 mmol) was dissolved in a 3 M solution of potassium hydroxide in 1-butanol (10 mL, 30 mmol). The resulting solution was heated under reflux for 3 h and allowed to cool. To the solution, 10 mL of a 1.6 M hydrochloric acid solution was added. The product was extracted with 3 x 40 mL of diethyl ether and washed with 3 x 40 mL of water. The organic extracts were dried over anhydrous sodium sulphate, decanted, and the solvent removed with a rotaryevaporator, producing a brown oil. Yield: 1.19 g (85.0%).
Safrole (1.40 g, 8.63 mmol) was dissolved in a 3 M solution of potassium hydroxide in 1-butanol (10 mL, 30 mmol). The resulting solution was heated under reflux for 3 h and allowed to cool. To the solution, 10 mL of a 1.6 M hydrochloric acid solution was added. The product was extracted with 3 x 40 mL of diethyl ether and washed with 3 x 40 mL of water. The organic extracts were dried over anhydrous sodium sulphate, decanted, and the solvent removed with a rotaryevaporator, producing a brown oil. Yield: 1.19 g (85.0%).
MDP2P (6) (Route 2)
A solution containing hydrogen peroxide (2.0 mL, 9.9 M, 20 mmol) and formic acid (10 mL, 23.6 M, 240 mol) was stirred at room temperature for 30 min in a 50 mL pear-shaped flask. Isosafrole (800 mg, 4.93 mmol) in 6 mL of acetone was added to the solution and stirred at room temperature for 16 h. The volatile components of the resulting solution were removed in vacuo, leaving a red residue. The residue was redissolved in 10 mL of methanol and 10 mL of 2.8 M sulphuric acid was added. The resulting solution was heated under reflux for 3 h and allowed to cool. The product was extracted with 3 x 40 mL of diethyl ether and washed with 40 mL of water and 40 mL of saturated sodium bicarbonate solution. The ether extracts were dried over anhydrous sodium sulphate, decanted and the solvent removed with a rotary evaporator, producing a brown oil. Yield: 538 mg (61.2 %).
A solution containing hydrogen peroxide (2.0 mL, 9.9 M, 20 mmol) and formic acid (10 mL, 23.6 M, 240 mol) was stirred at room temperature for 30 min in a 50 mL pear-shaped flask. Isosafrole (800 mg, 4.93 mmol) in 6 mL of acetone was added to the solution and stirred at room temperature for 16 h. The volatile components of the resulting solution were removed in vacuo, leaving a red residue. The residue was redissolved in 10 mL of methanol and 10 mL of 2.8 M sulphuric acid was added. The resulting solution was heated under reflux for 3 h and allowed to cool. The product was extracted with 3 x 40 mL of diethyl ether and washed with 40 mL of water and 40 mL of saturated sodium bicarbonate solution. The ether extracts were dried over anhydrous sodium sulphate, decanted and the solvent removed with a rotary evaporator, producing a brown oil. Yield: 538 mg (61.2 %).
Last edited by a moderator: